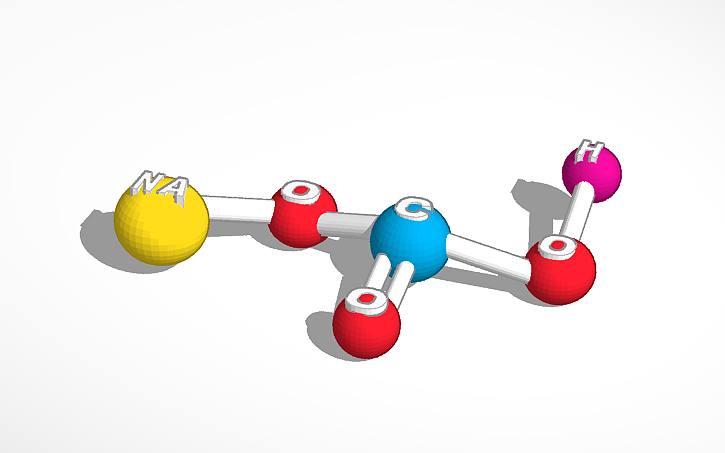
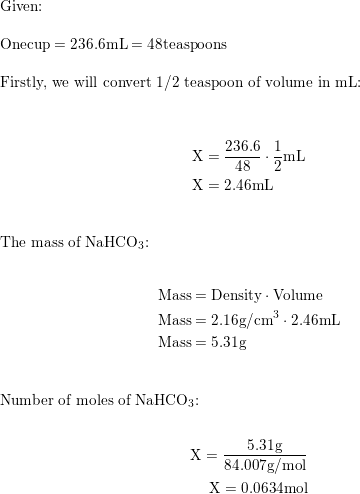Sodium bicarbonate, commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO₃. It is a salt… | Instagram

Water density science experiment || Water density changes when other substances dissolve in it - YouTube

Review of sodium bicarbonate (baking soda) particle size and its effect based on the method of production – Beroil Energy

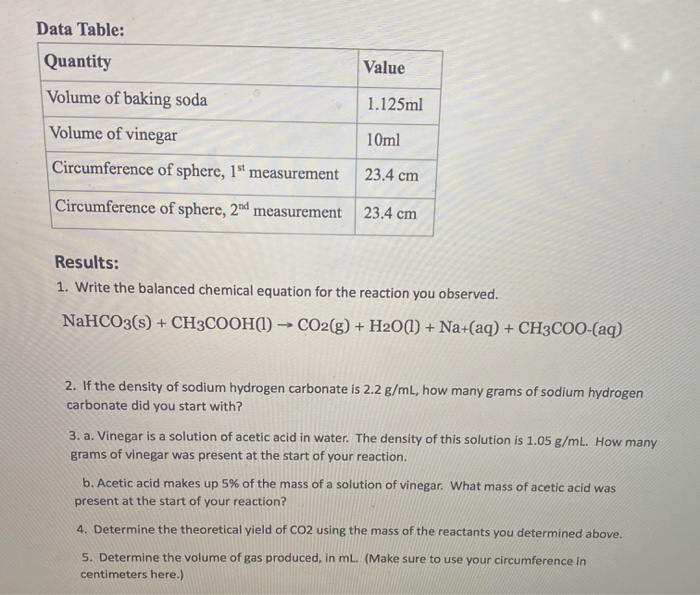
SOLVED: How many moles of baking soda are contributed by the 1/3 tsp. of baking soda in this recipe? Assume that the density of baking soda is 1 g/ml and the molecular

Wuxi Beitang Chemical Light Raw Material Co., Ltd Sodium bicarbonate(baking soda)Wuxi Beitang Chemical Light Raw Material Co., Ltd